Which particles determine the mass of an atom ?
The correct Answer is:protons, neutrons
Step by step video, text & image solution for Which particles determine the mass of an atom ? by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Related Playlists
- BOOK - DINESH PUBLICATIONCHAPTER - STRUCTURE OF ATOMEXERCISE - Multiple choice questions (Type-I)30videos
STRUCTURE OF ATOM
- BOOK - DINESH PUBLICATIONCHAPTER - STRUCTURE OF ATOMEXERCISE - Multiple choice questions (MCQs)11videos
STRUCTURE OF ATOM
- BOOK - DINESH PUBLICATIONCHAPTER - STRUCTURE OF ATOMEXERCISE - Value based questions3videos
STRUCTURE OF ATOM
- BOOK - DINESH PUBLICATIONCHAPTER - STATES OF MATTER : GASES AND LIQUIDSEXERCISE - Statement Type Question5videos
STATES OF MATTER : GASES AND LIQUIDS
- BOOK - DINESH PUBLICATIONCHAPTER - SURFACE CHEMISTRYEXERCISE - Ultimate Prepatarory Package16videos
SURFACE CHEMISTRY
Knowledge Check
- Question 1 - Select One
Soil particles determine its
AtextureBfield capacityCwater holding capacityDsoil flora - Question 1 - Select One
On the basis of Rutherford’s model of an atom, which sub- atomic particle is present in the nucleus of an atom?
AElectrons and neutronsBProtons and electyronsCProtons and neutronsDOnly electrons - Question 1 - Select One
Which of these sets of sub-atomic particles make up the nucleus of an atom?
AProtons and neutronsBProtons and electronsCElectrons and neutronsDElectrons, protons and neutrons
Similar Questions
Which particles determine the mass of an atom ?
View SolutionName the particles which actually determine the mass of an atom.
View SolutionOn the basis of Rutherford’s model of an atom, which sub- atomic particle is present in the nucleus of an atom?
View SolutionOn the basis of Rutherford’s model of an atom, which sub- atomic particle is present in the nucleus of an atom?
View SolutionParticle 1 experiences a perfectly collision with a staionary particle 2. Determine their mass ratio , if the particles move perpendicularly to each other.
View SolutionParticle 1 experiences a perfectly elastic collision with a stationary particle 2. Determine their mass ratio , if the particles fly symmetrically relative to the initial motion direction particle 1 with angle of divergence θ=60∘.
View SolutionThe diagram given below shows the sub-atomic particles present in the nucleus of atom X.
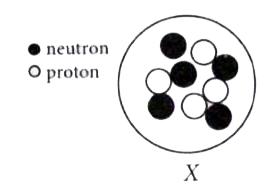
Which is the symbol for atom X ?View Solution

