Which one of the following is a compound most likely to have a dipole moment
A
CS2
B
H2S
C
SO3
D
SnCl4
Video Solution
Text Solution
The correct Answer is:B
H2S molecule has irregular geometry, because of presence of 2 lone pair of electrons or sulphur atom, and is therefore expected to have a dipole moment
|
Updated on:21/07/2023
Related Playlists
- BOOK - DINESH PUBLICATIONCHAPTER - CHEMICAL BONDING AND MOLECULAR STRUCTUREEXERCISE - REVISION279videos
CHEMICAL BONDING AND MOLECULAR STRUCTURE
- BOOK - DINESH PUBLICATIONCHAPTER - CHEMICAL BONDING AND MOLECULAR STRUCTUREEXERCISE - Comprehension M.C.Q24videos
CHEMICAL BONDING AND MOLECULAR STRUCTURE
- BOOK - DINESH PUBLICATIONCHAPTER - CARBOXYLIC ACIDS EXERCISE - BRAIN STORMING MULTIPLE CHOICE QUESTIONS (MCQS)13videos
CARBOXYLIC ACIDS
- BOOK - DINESH PUBLICATIONCHAPTER - CHEMICAL KINETICSEXERCISE - Additional Numerical Problems For Practice16videos
CHEMICAL KINETICS
Similar Practice Problems
- Question 1 - Select One
Which one of the following is a compound most likely to have a dipole moment
ACS2BCCl2F2CSO3DSnCl4 - Question 1 - Select One
Which one of the following isomeric compounds has the gretest dipole moment?
A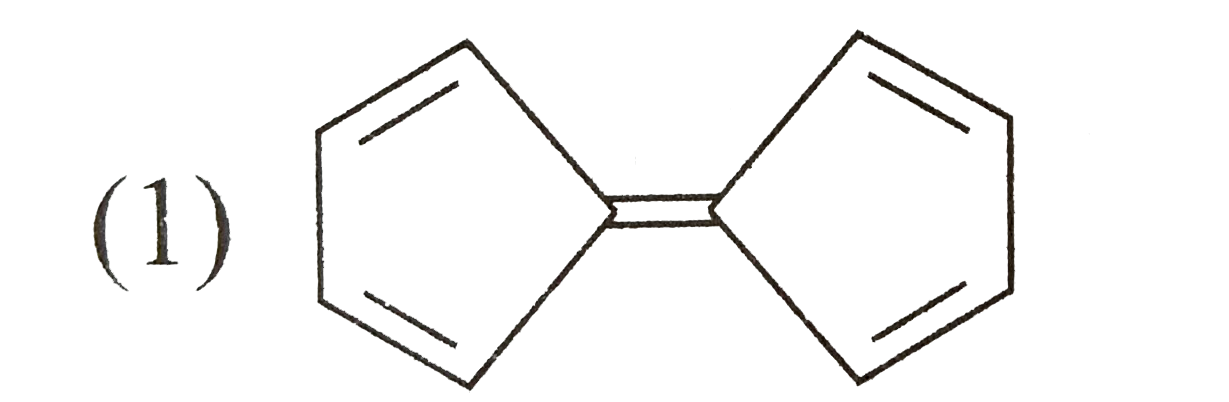 B
B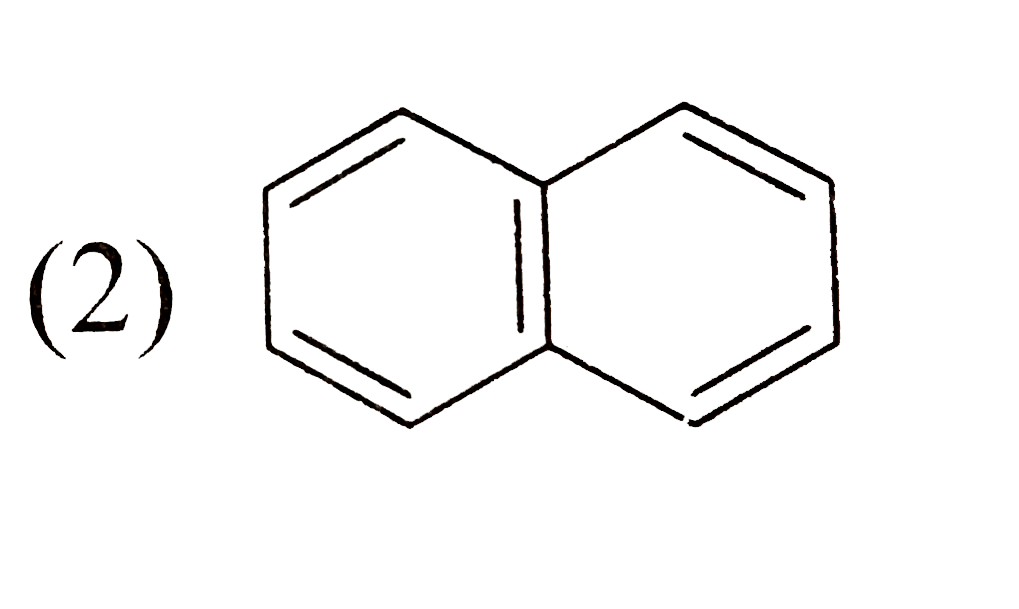 C
C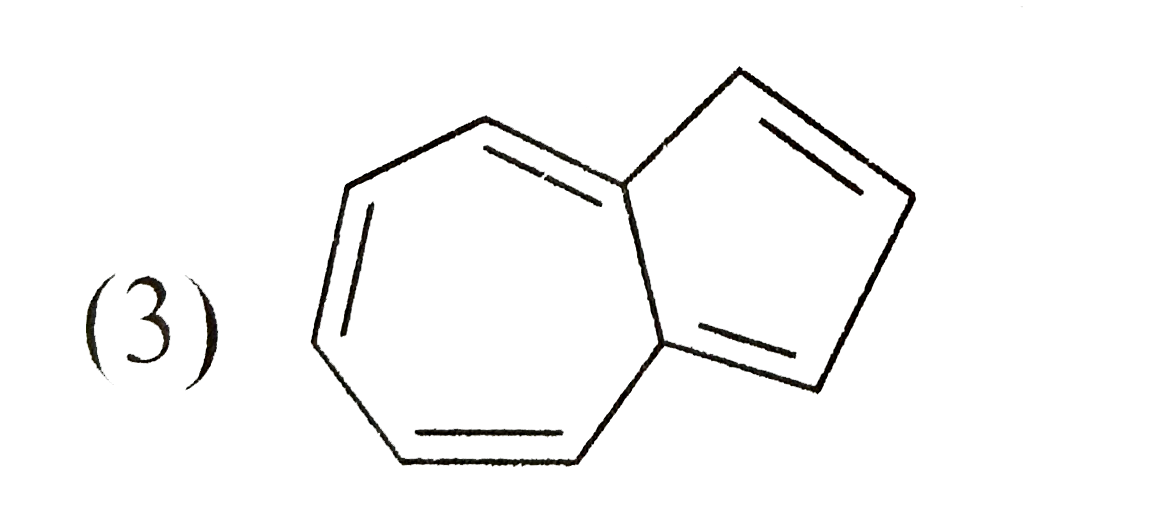 D
D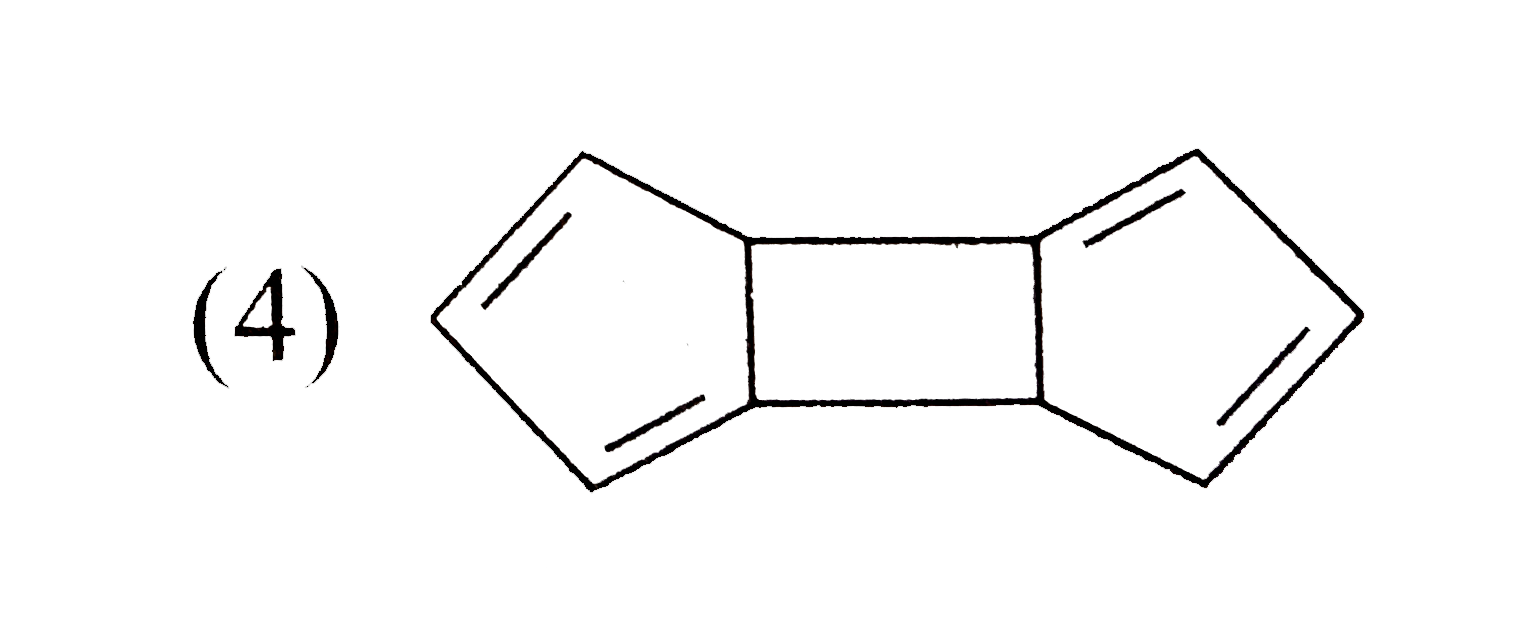
- Question 1 - Select One
Which of the following compounds would you expect to have a dipole moment ?
A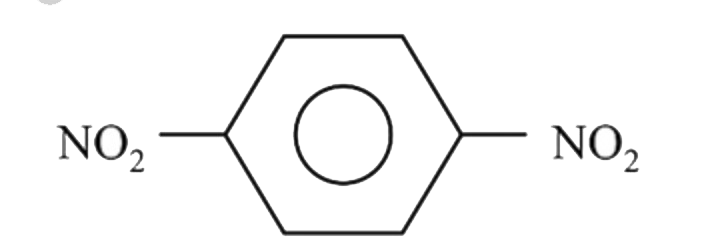 B
B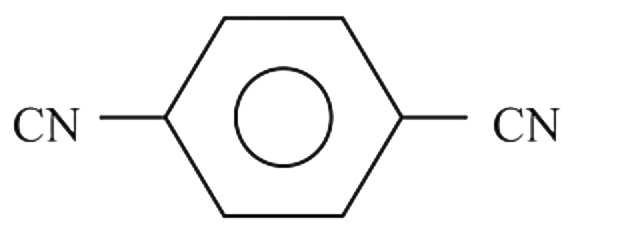 C
C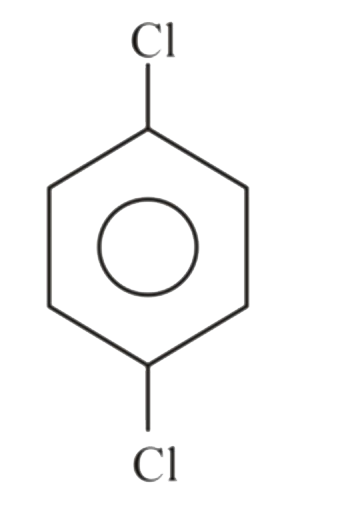 D
D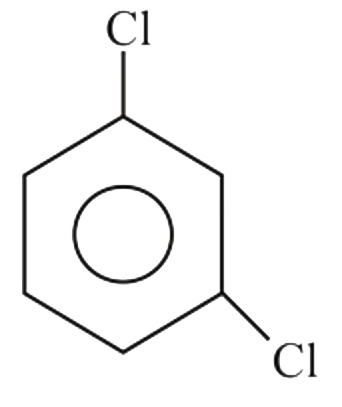
- Question 1 - Select One
Of the following compounds, which will have a zero dipole moment ?
Am-DinitrobenzeneBtrans-1,2-DichloroethyleneCcis-1,2-DichloroethyleneD1,4-Dichloroclohexane - Question 1 - Select One
Out of the following compounds which one will have zero dipole moment ?
AChloromethaneBDichloromethaneCTrichloromethaneDTetrachloromethane - Question 1 - Select One
Which of the following compounds will have the highest dipole moment ?
A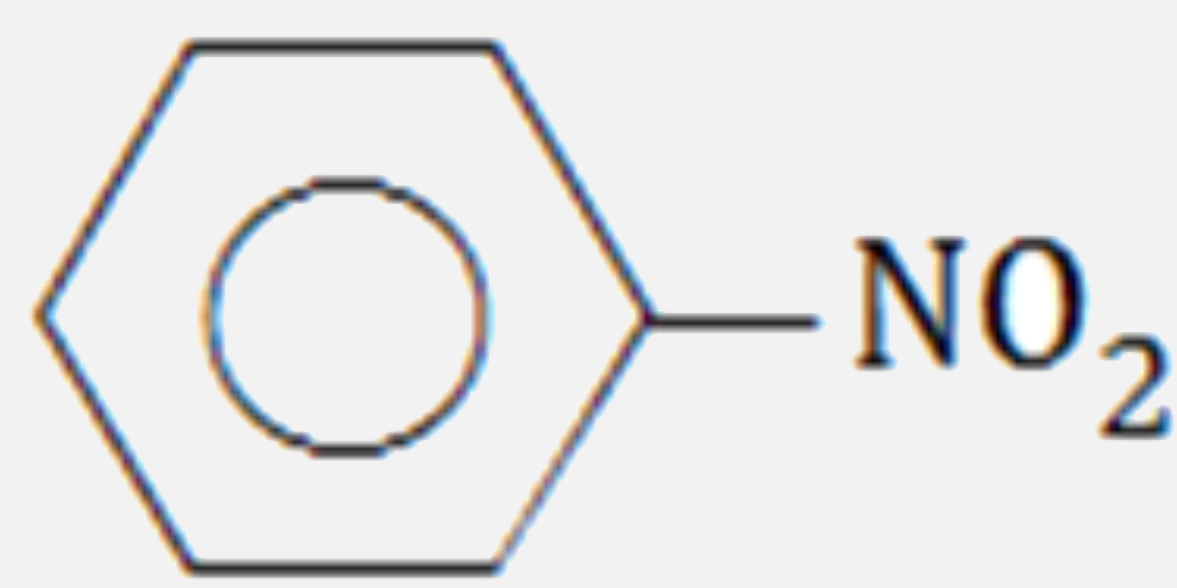 B
B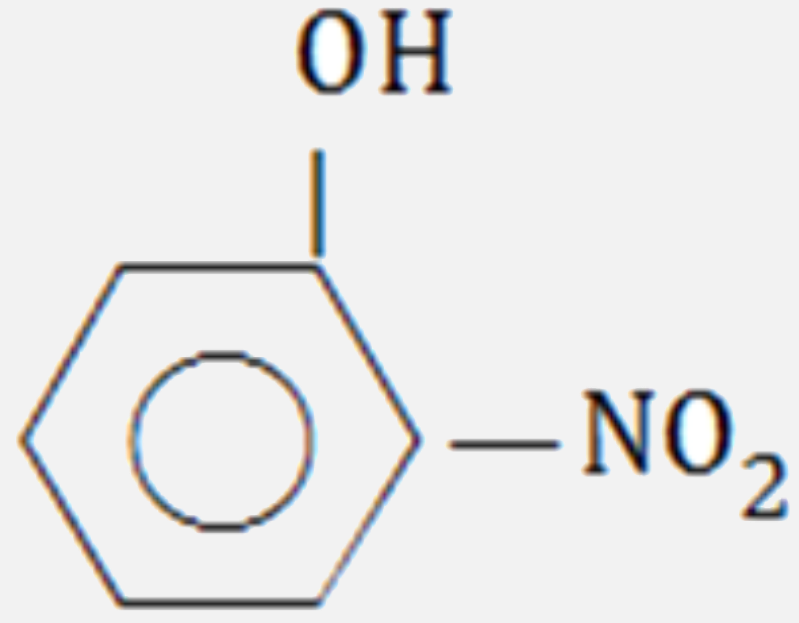 C
C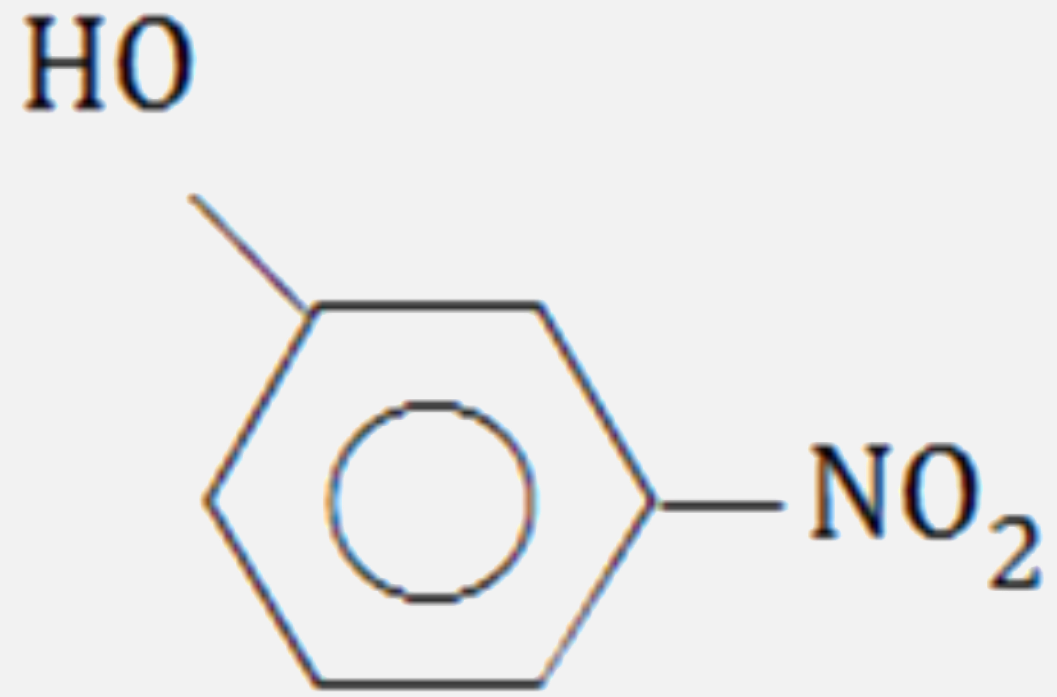 D
D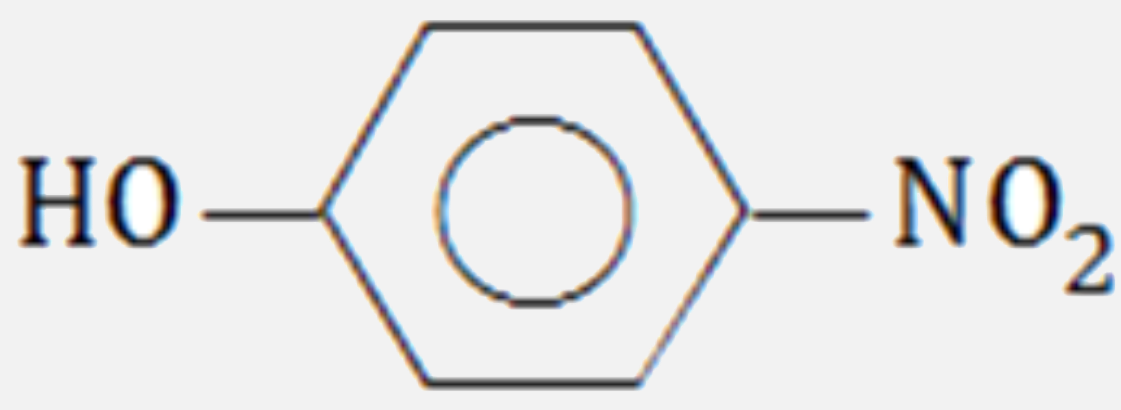
- Question 1 - Select One
Of the following compounds, which will have a zero dipole moment ?
A1 , 1 - DichloroethyleneBcis - 1 , 2 - DichloroethyleneCtrans - 1 , 2 - DichloroethyleneDNone of these compounds - Question 1 - Select One
Which of the following compounds will show highest dipole moment ?
 A(I)B(II)C(III)D(IV)
A(I)B(II)C(III)D(IV) - Question 1 - Select One
Which of the following pairs of molecules will have permanent dipole moment for both the members?
ASiF4 and NO2BNO2 and CO2CNO2 and O3DSiF4 and CO2

