Ligands are broadly classified into two classes classical and non-classical ligands, depending on their donor annd acceptor ability. Classical ligands form classical complexes while non-classical ligands form non-classical complex. Bonding mechanism in non-classical is called synergic bonding.
Q. Which is not π-acceptor ligand?
A
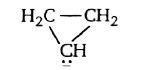
B
σ−C5H−5
C
PH3
D
B_(3)N_(3)H_(6)
Video Solution
Text Solution
The correct Answer is:A
No vacant 'd' or π∗ orbitals on 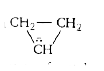
|
Updated on:21/07/2023
Related Playlists
- BOOK - VK JAISWALCHAPTER - CO-ORDINATION COMPOUNDSEXERCISE - ASSERTION-REASON TYPE QUESTIONS26videos
CO-ORDINATION COMPOUNDS
- BOOK - VK JAISWALCHAPTER - CO-ORDINATION COMPOUNDSEXERCISE - LEVEL 1202videos
CO-ORDINATION COMPOUNDS
- BOOK - VK JAISWALCHAPTER - CHEMICAL BONDING (BASIC)EXERCISE - Level 3 (Passive 11)6videos
CHEMICAL BONDING (BASIC)
- BOOK - VK JAISWALCHAPTER - d-BLOCK ELEMENTSEXERCISE - SUBJECTIVE PROBLEMS6videos
d-BLOCK ELEMENTS
Knowledge Check
- Question 1 - Select One
Ligands are broadly classified into two classes classical and non-classical ligands, depending on their donor annd acceptor ability. Classical ligands form classical complexes while non-classical ligands form non-classical complex. Bonding mechanism in non-classical is called synergic bonding.
Q. Synergic bonding is absent in:A[Mo(CO)6]B[Cr(CO)3(B3N3H6]C[Sc(CO)6]3+D[Ni(CN)4]4− - Question 1 - Select One
Ligands are broadly classified into two classes classical and non-classical ligands, depending on their donor annd acceptor ability. Classical ligands form classical complexes while non-classical ligands form non-classical complex. Bonding mechanism in non-classical is called synergic bonding.
Q. Synergic bonding is absent in:A[Mo(CO)6]B[Cr(CO)3(B3N3H6]C[Sc(CO)6]3+D[Ni(CN)4]4− - Question 1 - Select One
Ligands are broadly classified into two classes classical and non-classical ligands, depending on their donor annd acceptor ability. Classical ligands form classical complexes while non-classical ligands form non-classical complex. Bonding mechanism in non-classical is called synergic bonding.
Q. In compound [M(CO)n]x, the correct match for highest 'M-C' bond length for given M, n and z respectively:AM-Cr, n-6, z-0BM-V, n-6, z- −1CM-Ti, n-6, z- −2DM-Mn, n-6, z- +1 - Question 1 - Select One
Ligands are broadly classified into two classes classical and non-classical ligands, depending on their donor annd acceptor ability. Classical ligands form classical complexes while non-classical ligands form non-classical complex. Bonding mechanism in non-classical is called synergic bonding.
Q. In compound [M(CO)n]x, the correct match for highest 'M-C' bond length for given M, n and z respectively:AM-Cr, n-6, z-0BM-V, n-6, z- −1CM-Ti, n-6, z- −2DM-Mn, n-6, z- +1 - Question 1 - Select One or More
Which of the following chemical can act as non-classical ligand(s) ?
ACOBC2H4CNO+DPR3 - Question 1 - Select One or More
Which of the following chemical can act as non-classical ligand(s) ?
ACOBC2H4CNO+DPR3 - Question 1 - Select One or More
Which of the following chemical species can act a non-classical ligand(s) ?
ACompound Y has no counter ion.BC2H4CNO+DPR3 - Question 1 - Select One
The π acid ligand which uses it d-orbital during synergic bonding in its complex compound.
ACN−BPR3CNODN2

