Electron in hydrogen atom first jumps from third excited state to second excited state and then from second excited to the first excited state. The ratio of the wavelengths λ1,λ2 emitted in the two cases is
7/5
27/20
27/5
20/7
The correct Answer is:D
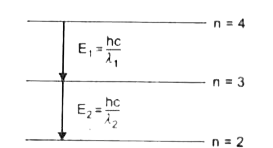
E1=hcλ1=13.6[1(3)2−1(4)2]........(i)
E2=hcλ2=13.6[1(2)2−1(3)2]......(ii)
dividing eq. (ii) by eq. (i)
λ1λ2=14−1919−116=207
Related Playlists
- BOOK - MODERN PUBLICATIONCHAPTER - ATOMS, MOLECULES AND NUCLEIEXERCISE - MCQ (Level -III)9videos
ATOMS, MOLECULES AND NUCLEI
- BOOK - MODERN PUBLICATIONCHAPTER - ATOMS, MOLECULES AND NUCLEIEXERCISE - Recent Competitive Questions40videos
ATOMS, MOLECULES AND NUCLEI
- BOOK - MODERN PUBLICATIONCHAPTER - ATOMS, MOLECULES AND NUCLEIEXERCISE - Recent Competitive Questions40videos
ATOMS, MOLECULES AND NUCLEI
- BOOK - MODERN PUBLICATIONCHAPTER - 15 MAGNETIC EFFECTS OF CURRENTSEXERCISE - Recent Competitive Questions31videos
15 MAGNETIC EFFECTS OF CURRENTS
- BOOK - MODERN PUBLICATIONCHAPTER - CURRENT ELECTRICITYEXERCISE - RECENT COMPETITIVE QUESTIONS32videos
CURRENT ELECTRICITY
Similar Questions
Electron in hydrogen atom first jumps from third excited state to second excited state and then form second excited state to first excited state. The ratio of wavelength λ1:λ2 emitted in two cases is
View SolutionElectron in hydrogen atom first jumps from third excited state to second excited state and then form second excited state to first excited state. The ratio of wavelength λ1:λ2 emitted in two cases is
View SolutionIn a hydrogen atom,electron moves from second excited state to first excited state and then from first excited state to ground state.The ratio of wavelengths is
View SolutionAn electron in hydrogen atom first jumps from second excited state to first excited state and then from first excited state to ground state. Let the ratio of wavelength, momentum and energy of photons emitted in these two cases be a, b and c respectively, Then
View SolutionAn electron in hydrogen atom first jumps form second excited state to first excited state and then form first excited state to ground state. Let the ratio of wavelength, momentum and energy of photons emitted in these two cases be a, b and c respectively, Then
View SolutionAn electron in hydrogen atom first jumps from second excited state to first excited state and then from first excited state to ground state. Let the ratio of wavelength, momentum and energy of photons emitted in these two cases be a, b and c respectively, Then
View SolutionWhen a hydrogen atom is excited from ground state to first excited state, then
View SolutionWhen a hydrogen atom is excited from ground state to first excited state, then
View SolutionThe ratio of energies of hydrogen atom in its first excited state to third excited state is
View SolutionThe ratio of energies of hydrogen atom in its first excited state to third excited state is
View Solution

