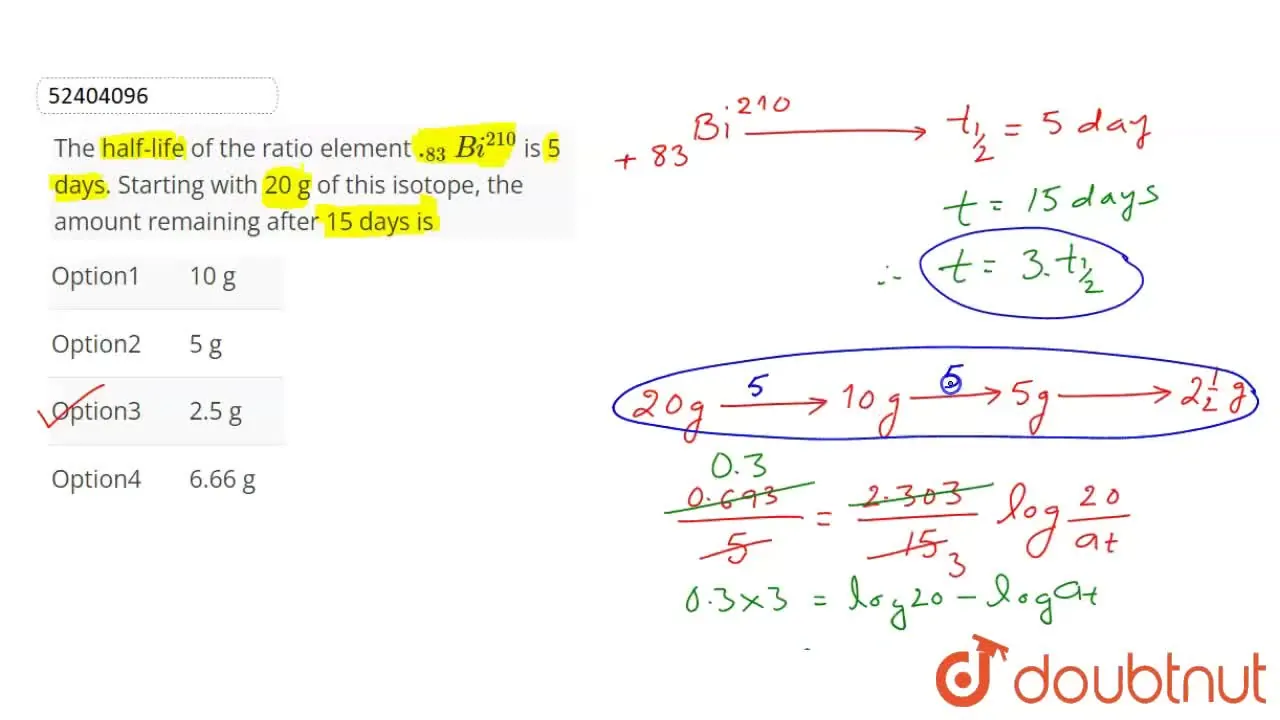
The half-life of the ratio element .83Bi210 is 5 days. Starting with 20 g of this isotope, the amount remaining after 15 days is
10 g
5 g
2.5 g
6.66 g
The correct Answer is:C
n=155=3,N=N02n=2023=208=2.5g
Related Playlists
- BOOK - ERRORLESS CHAPTER - NUCLEAR CHEMISTRY EXERCISE - Ordinary Thinking (Artificial transmutation )59videos
NUCLEAR CHEMISTRY
- BOOK - ERRORLESS CHAPTER - NUCLEAR CHEMISTRY EXERCISE - Ordinary Thinking (Isotopes-Isotones and Nuclear isomers)34videos
NUCLEAR CHEMISTRY
- BOOK - ERRORLESS CHAPTER - NUCLEAR CHEMISTRY EXERCISE - Ordinary Thinking (Causes of radioactivity and Group displacement law)66videos
NUCLEAR CHEMISTRY
- BOOK - ERRORLESS CHAPTER - NITROGEN CONTAINING COMPOUNDS EXERCISE - JEE (ADVANCED) 2018 MORE THAN ONE CHOICE CORRECT ANSWER1videos
NITROGEN CONTAINING COMPOUNDS
- BOOK - ERRORLESS CHAPTER - ORES, MINERALS AND METALLURGICAL EXTRACTIONEXERCISE - JEE ANSWER TPYE QUESTION (JEE Advanced) 2018) Numeric answer type question1videos
ORES, MINERALS AND METALLURGICAL EXTRACTION
Similar Questions
The t1/2 of a radionuclide is 8 hours. Starting with 40 g of the isotope, the amount in gm remainig after one day will be:
View SolutionHalf life of Bi210 is 5 days. If we start with 50,000 atoms of this isotope, the number of atoms left over after 10 days is
View SolutionA radioactive isotope has a half-life of 27 days. Starting with 4g of the isotope, what will be mass remaining after 75 days
View SolutionThe half -life period of .84Po210 is 140 days.
In how many days 1g of this isotope is reduced to 0.25g?View SolutionThe half life period of radio active isotope is 5 minutes. The fraction of isotope, that will be remaining after 30 minutes is
View SolutionA radioactive isotope has a half-life of 20 days. If 100 g of the substance is taken, the weight of the isotope remaining after 40 days is
View SolutionInitial amount of the radioactive element with half life 10 days is 16 g. What amount in gm of this element will remain after 40 days?
View SolutionHalf life of a radioactive element is 10 days. What percentage of the element will remain undecayed after 100 days?
View SolutionThe half-life period of radioactive element is 140 days. After 560 days, 1 g of element will reduce to
View SolutionThe half-life period of a radioactive element is 140 days. After 560 days, one gram of the element will reduce to
View Solution